CHEAT SHEET
TOP 10 RIGHT NOW
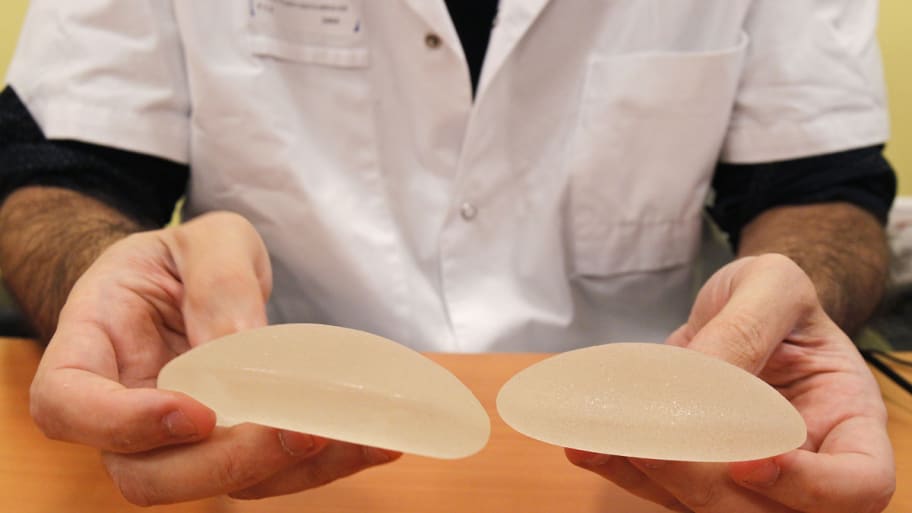
Michel Euler / AP Photo
You can thank your regulators for a change: the FDA expressed concern about defective French implants in 2000, a full 10 years before European regulators began to worry. The FDA sent an inspector to the implants’ manufacturer, Poly Implant Prothese, and then sent its founder a letter saying the implants were “adulterated” and listing at least 11 problems with the manufacturing process. PIP, it turns out, was using computer-grade silicone in the implants. Thousands of European women have received them, and the French government has recommended that its citizens have them removed due to their high rupture rate.