A new Alzheimer’s drug has been shown to significantly slow the disease’s progression in patients—offering a promising and potentially powerful tool in the fight against the neurodegenerative disorder.
In a study of more than 1,700 patients published Monday in the journal JAMA, researchers at pharmaceutical company Eli Lilly show how the drug—called donanemab—is effective in attacking plaque in the brain made of a protein called amyloid, which scientists believe is responsible for Alzheimer’s.
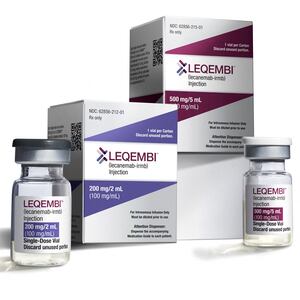
In trials, donanemab slowed cognitive decline in patients by 35 percent when compared to a control group that received a placebo. For comparison, the recently FDA-approved Alzheimer’s drug Leqembi was shown to reduce cognitive decline by 27 percent.
While the results show a lot of promise, the authors note that it’s most effective for patients at the earliest stages of the disease. The drug is now expected to receive approval from the U.S. Food and Drug Administration (FDA).
“The general belief is that treating Alzheimer disease at the earliest disease stage is likely to result in more clinically meaningful effects,” the study authors wrote. They later added that the findings suggest that “a greater benefit from amyloid-lowering therapies may occur when initiated at an earlier disease stage.”
Donanemab works similarly to other drug therapies for Alzheimer’s that target amyloid proteins such as Aduhelm and Leqembi. However, the study showed that it was even more effective in removing the plaque from brains than the other two treatments. Patients who took donanemab also had a 40 percent lower risk of progressing from mild to moderate dementia.
Gil Rabinovici, the director of the Alzheimer’s Disease Research Center and wasn’t involved in the study, wrote in an accompanying editorial in JAMA that the drug is “the opening chapter in a new era of molecular therapies for Alzheimer’s disease and related neurodegenerative disorders.”
However, both the drug and the study had several limitations. For one, patients who had more advanced Alzheimer’s showed little to no benefit in treatment. The drug also has a number of potentially serious side effects such as amyloid-related imaging abnormalities (ARIA), which can result in brain swelling and even small bleeds in the brain. ARIA occurred in 3.7 percent of patients and resulted in three deaths.
The study only lasted 76 weeks, which means researchers still don’t understand the long-term implications of the treatment. However, the authors wrote that there is an ongoing study extension underway. More research is needed to understand the full effects of the treatment.
The Eli Lilly team noted that there was also a lack of diversity in the study participants, with 91.5 percent of them being white. As such, researchers are limited in understanding how donanemab impacts people of color, which is a massive barrier to fighting the disease considering that Black and Latino patients show higher rates of dementia.
The drug is also coming in the midst of controversy when it comes to Alzheimer’s research. More and more experts have begun to call into question whether or not amyloid proteins are responsible for the disease. The FDA also came under fire after it approved a number of experimental Alzheimer’s drugs in the past two years despite protests from scientific advisory panels and experts at large.
Still, the study’s results offer promise and hope for more than six million Americans living with Alzheimer’s each day and marks a major turning point in treating the disease. The drug and its approach to treating amyloid plaque could even lead to newer, more effective treatments—allowing those with early stage dementia to live their normal, day-to-day lives with their cognition and memories intact for at least a little longer.