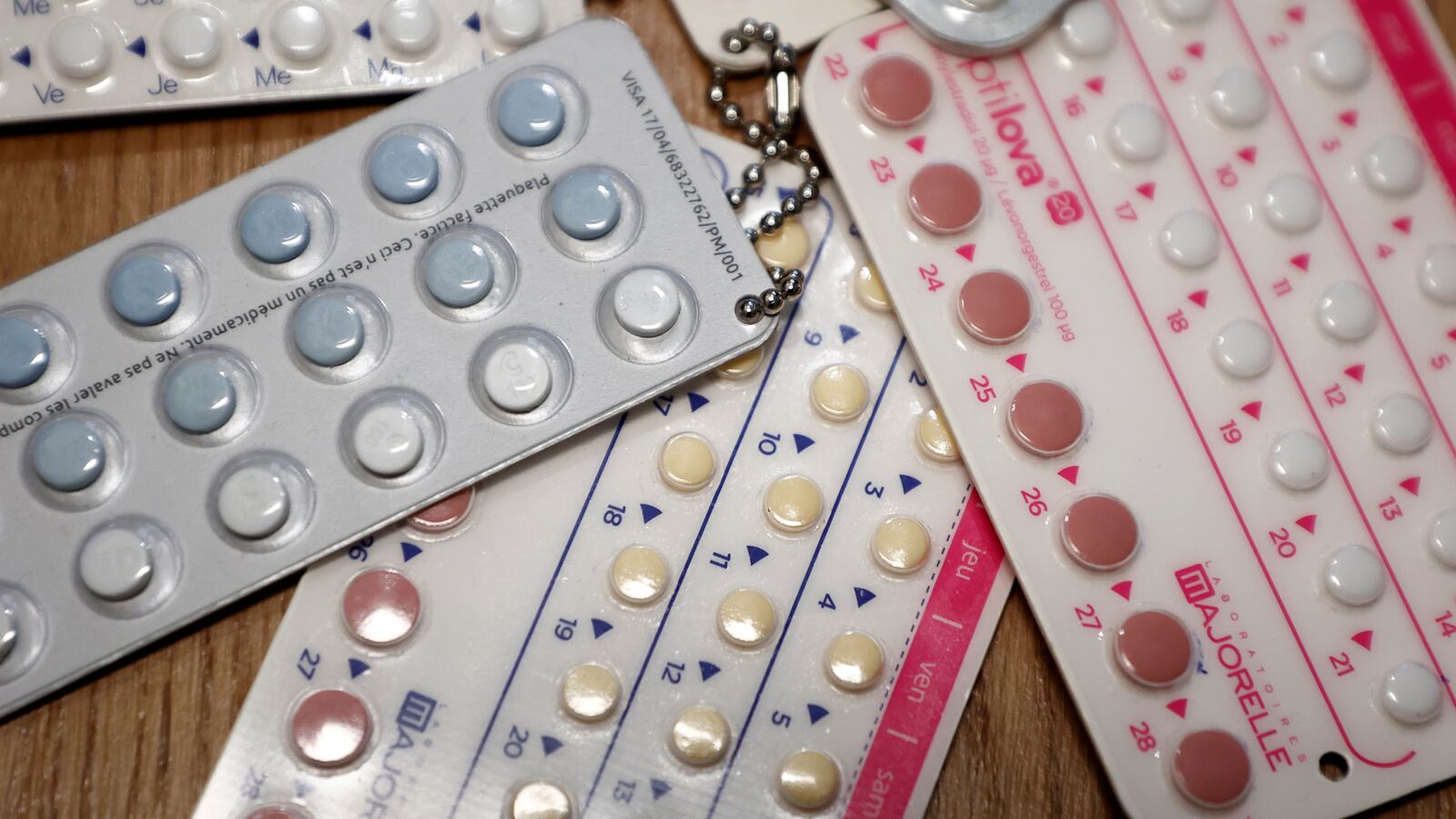
Though millions of Americans have used hormone-based birth control pills for decades, the medication still requires a prescription. That may change, as a pharmaceutical company has asked the FDA for approval to sell its pill over the counter in the U.S. for the first time. The Associated Press reports that French-based HRA Pharma sent its application to the FDA on Monday. The timing of the request, the company said, is unrelated to the recent overturning of Roe v. Wade and was based on years of research. When prescribing birth control, health-care professionals check patients for conditions that increase the risk of exceedingly rare blood clots, but HRA says that its research shows women can safely self-screen. The request places the FDA under political pressure, as the decision is closely tied to the debate about reproductive rights. HRA Pharma expects the agency to reach a decision in the first half of next year.
Read it at Associated Press