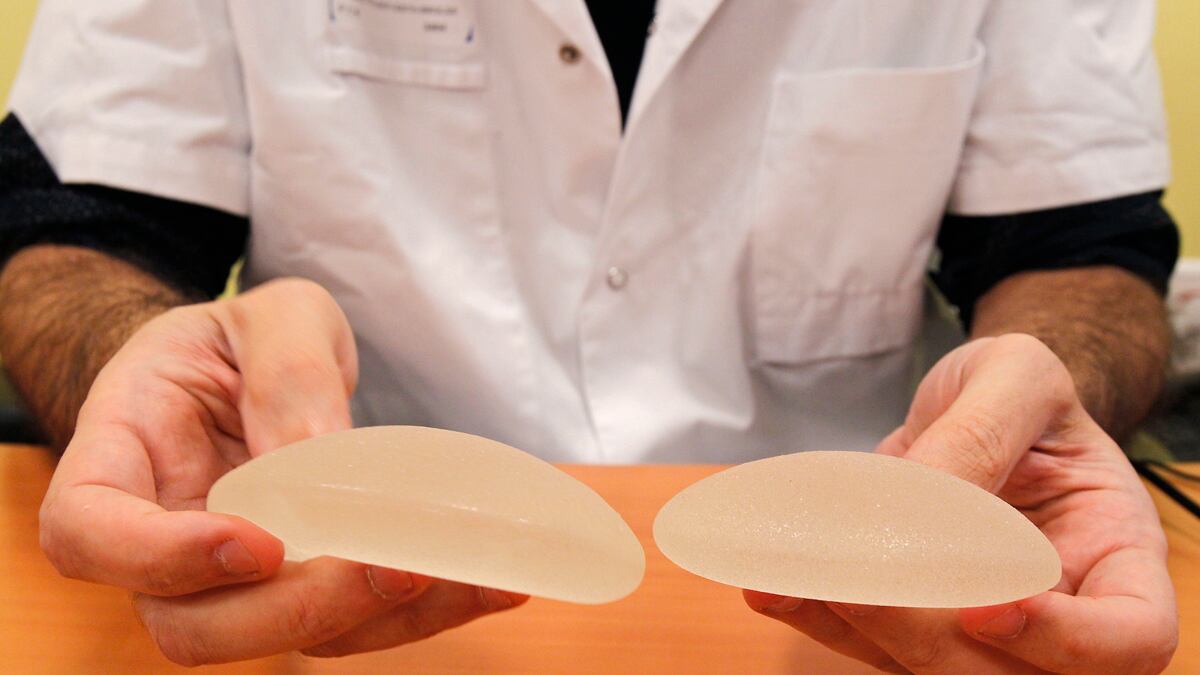
You can thank your regulators for a change: the FDA expressed concern about defective French implants in 2000, a full 10 years before European regulators began to worry. The FDA sent an inspector to the implants’ manufacturer, Poly Implant Prothese, and then sent its founder a letter saying the implants were “adulterated” and listing at least 11 problems with the manufacturing process. PIP, it turns out, was using computer-grade silicone in the implants. Thousands of European women have received them, and the French government has recommended that its citizens have them removed due to their high rupture rate.
Read it at Reuters