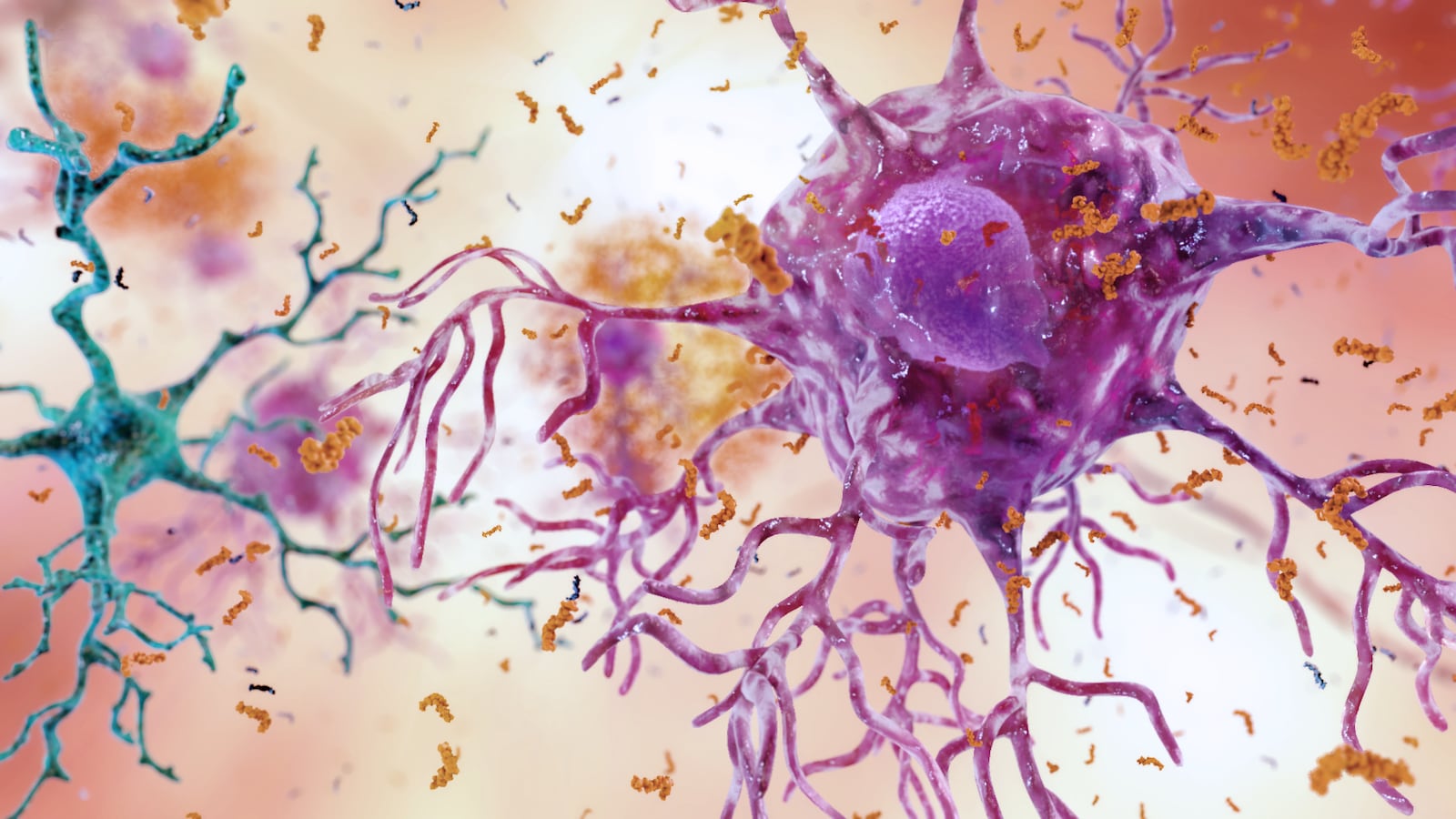
Alzheimer’s has always been a poorly understood disease, and that’s raised huge challenges in finding viable treatments. Scientists are even exploring unorthodox options, like old drugs prescribed for very different conditions. They’ve found a very surprising candidate, in the form of a 50-year-old diuretic.
On Monday, researchers showed that the drug bumetanide, normally used to manage fluid retention for conditions like hypertension, led to a reversal of Alzheimer’s symptoms in laboratory mice as well as human brain cells. And through a study of the health records of millions of patients, those same researchers found that people over 65 who took bumetanide regularly were 35–75 percent less likely to get diagnosed with Alzheimer’s.
The findings, published in Nature Aging, hinges around the gene APOE, which mediates fat metabolism in mammals but is also known to be a major risk factor for Alzheimer’s. Through an analysis of APOE gene records in Alzheimer’s patients, along with the data of 1,300 existing drugs, the researchers found that bumetanide might be associated with reversing the effects of altered APOE genes. The drug’s mechanisms for changing fluid retention in cells also plays a role in how neurons in the brain trigger electrical signaling, so it seems worth exploring.
It seems the researchers’ intuition about the drug was right. And Bumetanide’s potential to treat Alzheimer’s is bolstered by the fact that it is already FDA-approved, and has a safety record that stretches back for decades. “This is a drug with a well-established safety record, so it could reach patients much faster than typical drug development takes,” Gladstone Institute researcher and study coauthor Yadong Huang said in a statement.
That’s great news for getting a clinical trial started to truly test whether bumetanide can help combat Alzheimer’s. Huang and his team are already in advanced talks with other medical centers to get one started.